Describe How Hydrogen and Oxygen Form Water
The image above depicts water molecules. These bond together to form water as illustrated in Figure 1.

Ozone The Other Oxygen A Brief Discussion Quirky Science Oxygen Bond Length Intermolecular Force
Hydrogen bonding is an attractive force between two molecules that relies on the slight polarity of the O-H O-F or O-N bond.

. A water molecule consists of two atoms of hydrogen linked by covalent bonds to the same atom of oxygen. Two hydrogen atoms each share their 1 electron with oxygen to form two covalent bonds and make a water molecule H 2 O. A hydrogen atom has 1 electron in its outer shell.
Kattyahto8 and 63 more users found this answer helpful. Two or more atoms may bond with each other to form a molecule. Describe how hydrogen and oxygen form water.
When hydrogen burns in air it combines with atmospheric oxygen to form water. When molecular hydrogen H 2 and oxygen O 2 are combined and allowed to react together energy is released and the molecules of hydrogen and oxygen can combine to form either water or hydrogen. Chemistry 22062019 0110 isabellemdeakin.
Can someone me with these questions plz. 1 Get Other questions on the subject. Remember that a water molecule contains two hydrogen atoms and one oxygen atom.
This is a picture of a water molecule. Grant Mason from the BYU Department of Physics and Astronomy demonstrates the reaction of hydrogen and oxygen to form water. Industrial methods for producing hydrogen use an inexpensive reducing agent such as hot iron carbon.
Since hydrogen and oxygen are undergoing a chemical change to become chemically bonded together producing H2O the. When molecules of water vapor in the air come in contact with a cold can of soft drink they lose energy slow downand form a liquid due to decrease in. When two hydrogens and an oxygen share.
Atoms of oxygen are electronegative and attract the shared electrons in their covalent bonds. Mixtures of hydrogen and oxygen or hydrogen and air can be explosive when the two gases are present in. By sharing the two electrons where the shells touch each hydrogen atom can count 2 electrons in its outer shell.
2H 2 O 2 2H 2 O. We also acknowledge previous National Science Foundation support under grant numbers. So one molecule of water is formed when two atoms of hydrogen combine with one atom of oxygenAs both hydrogen and oxygen are electronegative in naturethey share their valence electron As a result of which two single covalent bonds are formed to form 1 molecule of water.
Describe how hydrogen and oxygen form water. Converting water to hydrogen and oxygen and then letting them recombine is one way of purifying it but probably one of the least efficient ways. Consequently the electrons in the water molecule spend slightly more time around the oxygen atomic center and less time around the hydrogen atomic centers.
1 Show answers Another question on Chemistry. This is because the oxygen atom in addition to forming bonds with the hydrogen atoms also carries two pairs of unshared electrons. As Mathew said look at the Standard Gibbs free energy of formation.
Water forms when there is enough energy to break the molecular bonds within the Hydrogen and Oxygen diatoms they then combine and form water. Due to the electronegativity difference between the atom pairs mentioned electrons are unevenly shared across the covalent bond. The LibreTexts libraries are Powered by MindTouch and are supported by the Department of Education Open Textbook Pilot Project the UC Davis Office of the Provost the UC Davis Library the California State University Affordable Learning Solutions Program and Merlot.
The purest hydrogen is produced by electrolysis of water however this process is energy intensive and is not economical for large scale production. Since oxygen is more electronegative than hydrogen the. Water does not have two oxygen atoms has one and one hydrogen has two hence the molecular formula of water is H2O.
Hydrogen can only form 1 bond. I assume you are referring to the water molecule H_2O. Use your own words and do not copy and paste.
The key to understanding waters chemical behavior is its molecular structure. Thats why hydrogen is not a new energy source just another way of carrying energy around Of course that also means that hydrogen is not a new source of water either. Describe How Hydrogen And Oxygen Form WaterThe familiar water molecule H2O consists of two hydrogen atoms and one oxygen atom.
A water molecule consists of two hydrogen atoms bonded to an oxygen atom and its overall structure is bent. H_2O is indeed a polar molecule because it has polar bonds between the oxygen atom and the two hydrogen atoms and because its molecular geometry allows for the two bonds dipole moments to add to each other instead of cancelling out. Small amounts of hydrogen are conventionally prepared in the laboratory by reaction of dilute acid with an active metal such as zinc.
Hydrogen oxygen water. The flame is almost colourless.

Hydrogen And Oxygen Combine In The Ratio Of 1 8 By Mass To Form Water
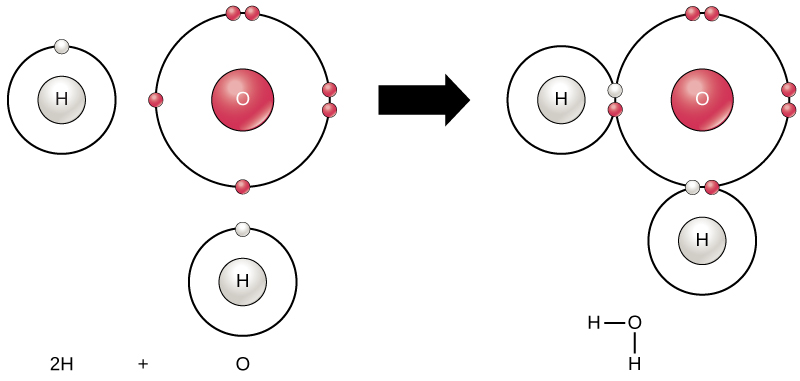
Chemical Reactions And Molecules Biology For Majors I

What S Up With That The Mysterious Effect That Makes Hot Water Freeze Faster Than Cold Hydrogen Bond Chemistry Lessons Water Molecule

What Is Matter What Is Matter Kids Discover Matter

Simple Diagram Showing Electrolysis Of Water To Make Hydrogen And Oxygen Gas Oxygen Free Energy Projects Chemistry Classroom

Multimedia A Catalyst And The Rate Of Reaction Chapter 6 Lesson 5 Middle School Chemistry
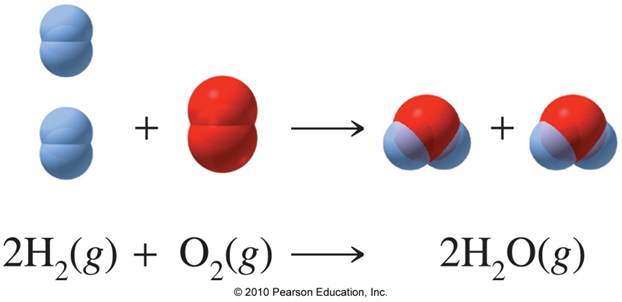
How Many Grams Of Water Can Be Produced The Combination Of 8 Grams Of Oxygen And 8 Grams Of Hydrogen Socratic

Welcome To Learnapchemistry Com Chemistry Classroom Teaching Chemistry Worksheet Template
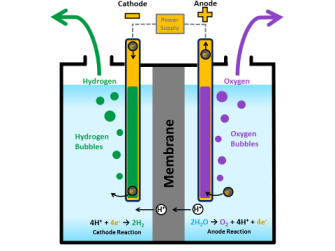
Limitless Hydrogen Energy Breakthrough Seen In Separating H From H2o Big Think

A Water Molecule Contains Two Atoms Of Hydrogen H And One Atom Of Oxygen O Scientists Have Molecules Science For Kids Water Molecule

Water Synthesis Reaction Students Britannica Kids Homework Help

Four Elements Make Up Most Living Things Including You The Elements Are Carbon Hydrogen Oxygen And Nit Science Lessons Science Chemistry Science Biology

How To Balance H2o H2 O2 Decomposition Of Water Youtube

Water Decomposes To Form Hydrogen Gas And Oxygen Gas H 2 O L H 2 G O 2 G 2 H 2 O L 2 H 2 G O 2 G Can You Guess Which Tube Holds Ppt Download

How To Balance H2 O2 H2o Youtube

Water Molecules Poster Zazzle Com Water Molecule Molecules Chemistry Classroom

Ozone The Other Oxygen A Brief Discussion Quirky Science Intermolecular Force Ozone Science

Water A Level Notes Water Molecule Teaching Chemistry Water Atom
Comments
Post a Comment